A physics perspective on wound healing
Scientists from UNIGE and UZH have used a statistical physics approach to identify the lengthscales of key intercellular interactions which govern tissue healing.
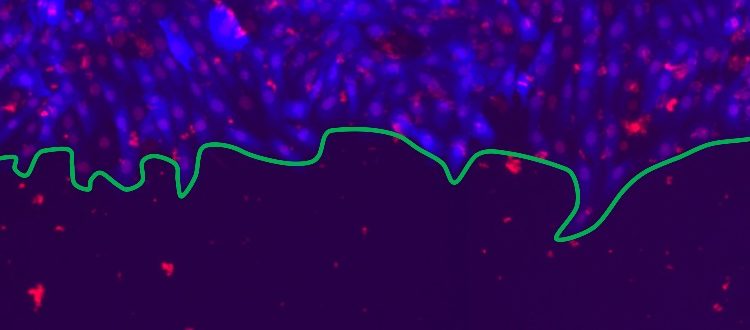
In material physics understanding how systems interact across the interfaces separating them is of central interest. But can physical models clarify similar concepts in living systems, such as cells? Physicists at the University of Geneva (UNIGE), in collaboration with the University of Zurich (UZH), used the framework of disordered elastic systems to study the process of wound healing – the proliferation of cell fronts which eventually join to close a lesion. Their study identified the scales of the dominant interactions between cells which determine this process. The results, published in the journal Scientific Reports, will allow better analysis of cell front behaviour, in terms of both wound healing and tumour development. In the future, this approach may offer personalised diagnostics to classify cancers and better target their treatment, and identify new pharmacological targets for transplantation.
By focusing on macroscopic properties of large datasets, statistical physics makes it possible to extract an overview of system behaviour independent of its specific microscopic character. Applied to biological elements, such as the cell fronts bordering a wound, this approach makes it possible to identify the various interactions which play a defining role during tissue growth, differentiation, and healing, but above all to highlight their hierarchy at the different scales observed. Patrycja Paruch, professor in the Department of Quantum Matter Physics at the UNIGE Faculty of Science, explains: “For cancer tumour invasion, or in the event of a wound, cell front proliferation is crucial, but the speed and morphology of the front is highly variable. However, we believe that only a few dominant interactions during this process will define the dynamics and the shape – smooth or rough, for example – of the cell colony edge. Experimental observations across multiple lengthscales to extract general behaviours can allow us to identify these interactions in healthy tissue and diagnose at what level pathological changes can occur, to help combat them. This is where statistical physics comes in.”
The many scales of wound healing
In this multidisciplinary study, the UNIGE physicists collaborated with the team of Professor Steven Brown from the UZH. Using rat epithelial cells, they established flat colonies (2D) in which the cells grow around a silicone insert, subsequently removed to mimic an open lesion. The cell fronts then proliferate to fill the opening and heal the tissue. “We reproduced five possible scenarios by ‘handicapping’ the cells in different ways, in order to see what impact this has on wound healing, i.e. on the speed and roughness of the cell front”, explains Guillaume Rapin, a researcher in Patrycja Paruch’s team. The idea is to see what happens in normal healthy tissue, or when processes such as cell division and communication between neighbouring cells are inhibited, when cell mobility is reduced or when cells are permanently pharmacologically stimulated. “We took some 300 images every four hours for about 80 hours, which allowed us to observe the proliferating cell fronts at very different scales”, continues Guillaume Rapin. “By applying high-performance computational techniques, we were able to compare our experimental observations with the results of numerical simulations”, adds Nirvana Caballero, another researcher in Patrycja Paruch’s team.
Zooming out for greater effect
The scientists observed two distinct roughness regimes: at less than 15 micrometres, below the size of a single cell, and between 80 and 200 micrometres, when several cells are involved. “We have analysed how the roughness exponent evolves over time to reach its natural dynamic equilibrium, depending on the pharmacochemical conditions we have imposed on the cells, and how this roughness increases depending on the scale at which we look”, emphasises Nirvana Caballero. “In a system with a single dominant interaction, we expect to see the same roughness exponent at all scales. Here, we see a changing roughness if we look at the scale of one cell or of ten cells.”
The Geneva and Zurich teams revealed only minor variations in the roughness exponent below 15 micrometres, whatever the conditions imposed on the cell fronts. On the other hand, they found that between 80 and 150 micrometers, the roughness is altered by all pharmacological inhibitors, significantly reducing the roughness exponent. Moreover, they observed that proliferation speed varied greatly between the different pharmacochemical conditions, slowing when cell division and motility were inhibited, and accelerating when cells were stimulated. “More surprisingly, the fastest proliferation speed was achieved when gap-junction communication between cells was blocked”, says Guillaume Rapin. This observation suggests that such communication may be targeted in future therapies, either to promote healing of burns or wounds, or to slow cancer tumour invasion.
These results show that medium-scale interactions play a crucial role in determining the healthy proliferation of a cell front. “We now know at what scale biologists should look for problematic behaviour of cell fronts, which can lead to the development of tumours”, says Nirvana Caballero. Now scientists will be able to focus on these key lenghtscales to probe tumour cells fronts, and directly compare their pathological interactions with this of healthy cells.
Press release in French
Read the article in Scientific Reports
Paruch group web site
Contacts: Prof. Patrycja Paruch, Guillaume Rapin, Dr Nirvana Caballero